Tissues and Organs
Blood flow in vessels, biophysics of organ systems, biomechanics.
Blood flow in vessels, biophysics of organ systems, biomechanics.
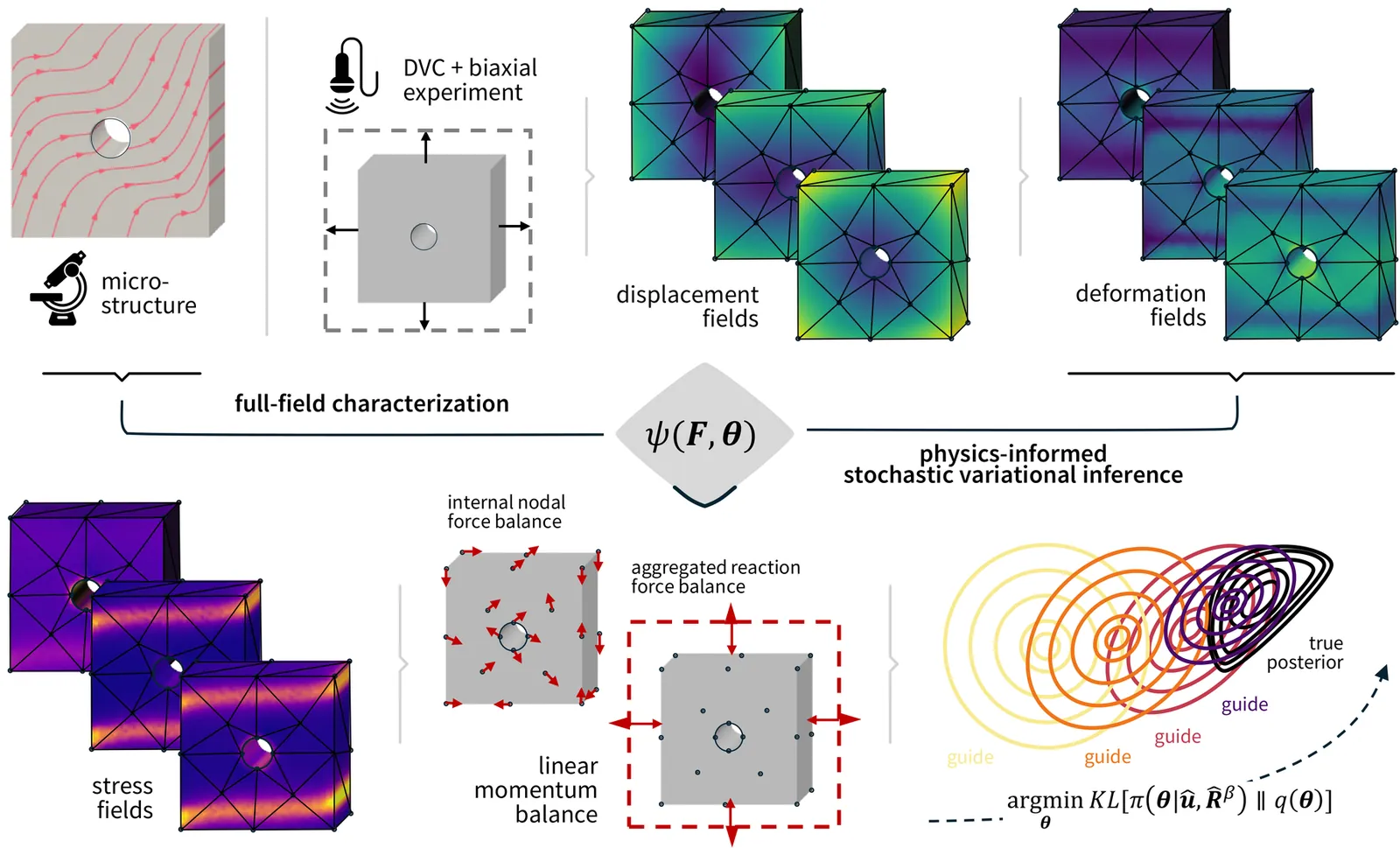
Fully capturing this behavior in traditional homogenized tissue testing requires the excitation of multiple deformation modes, i.e. combined triaxial shear tests and biaxial stretch tests. Inherently, such multimodal experimental protocols necessitate multiple tissue samples and extensive sample manipulations. Intrinsic inter-sample variability and manipulation-induced tissue damage might have an adverse effect on the inversely identified tissue behavior. In this work, we aim to overcome this gap by focusing our attention to the use of heterogeneous deformation profiles in a parameter estimation problem. More specifically, we adapt EUCLID, an unsupervised method for the automated discovery of constitutive models, towards the purpose of parameter identification for highly nonlinear, orthotropic constitutive models using a Bayesian inference approach and three-dimensional continuum elements. We showcase its strength to quantitatively infer, with varying noise levels, the material model parameters of synthetic myocardial tissue slabs from a single heterogeneous biaxial stretch test. This method shows good agreement with the ground-truth simulations and with corresponding credibility intervals. Our work highlights the potential for characterizing highly nonlinear and orthotropic material models from a single biaxial stretch test with uncertainty quantification.
 2509.16328
2509.16328Large-language models (LLMs) are rapidly being applied to radiology, enabling automated image interpretation and report generation tasks. Their deployment in clinical practice requires both high diagnostic accuracy and low inference latency, which in turn demands powerful hardware. High-performance graphical processing units (GPUs) provide the necessary compute and memory throughput to run large LLMs on imaging data. We review modern GPU architectures (e.g. NVIDIA A100/H100, AMD Instinct MI250X/MI300) and key performance metrics of floating-point throughput, memory bandwidth, VRAM capacity. We show how these hardware capabilities affect radiology tasks: for example, generating reports or detecting findings on CheXpert and MIMIC-CXR images is computationally intensive and benefits from GPU parallelism and tensor-core acceleration. Empirical studies indicate that using appropriate GPU resources can reduce inference time and improve throughput. We discuss practical challenges including privacy, deployment, cost, power and optimization strategies: mixed-precision, quantization, compression, and multi-GPU scaling. Finally, we anticipate that next-generation features (8-bit tensor cores, enhanced interconnect) will further enable on-premise and federated radiology AI. Advancing GPU infrastructure is essential for safe, efficient LLM-based radiology diagnostics.
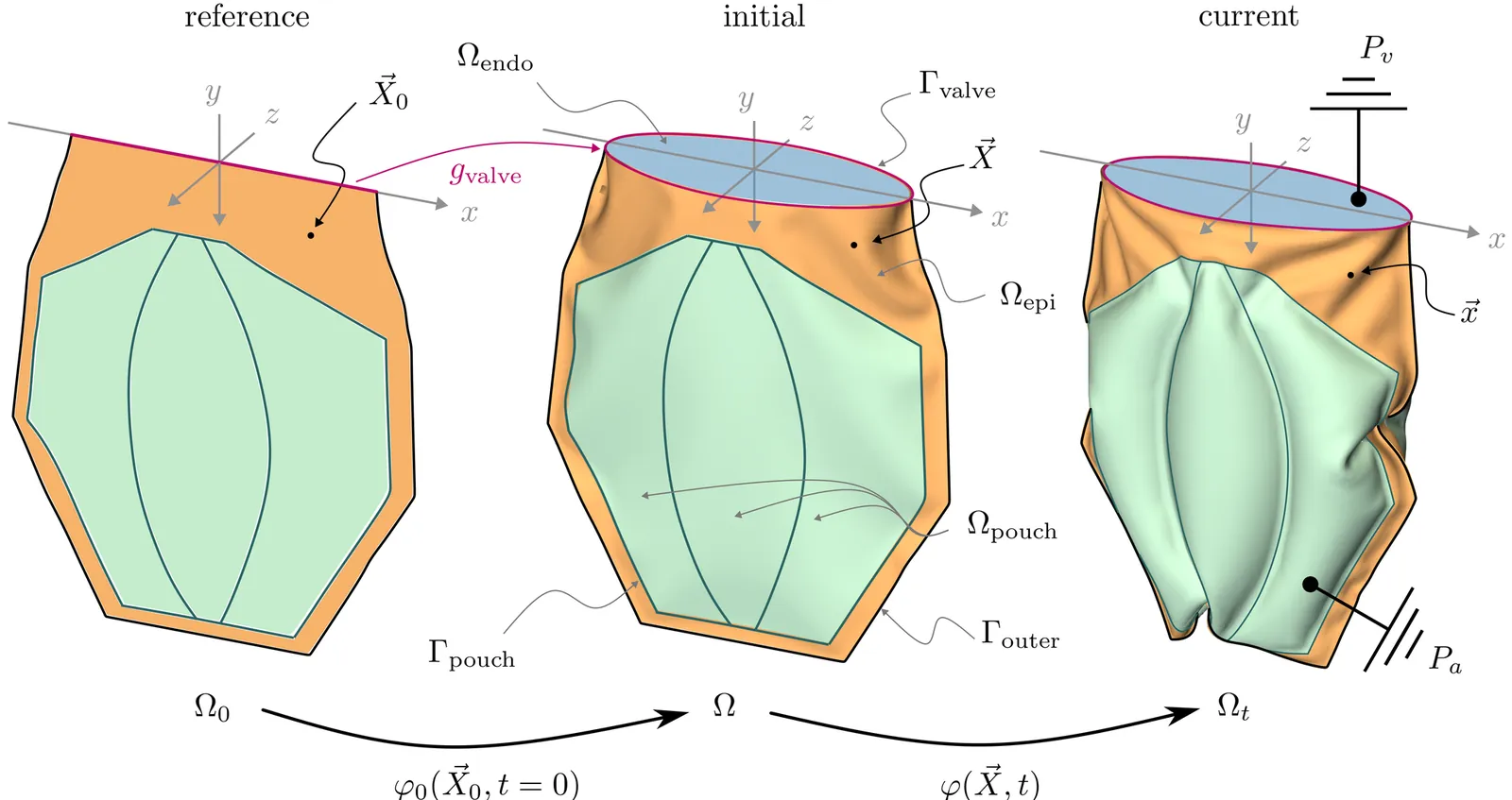
Fabric-based soft robots combine high load-carrying capacity, efficiency, and low weight with the ability to bend, twist, contract, or extend with ease, making them promising candidates for biomedical applications such as soft total artificial hearts. While recent experiments have demonstrated their potential, predictive numerical models are urgently needed to study their complex mechanics, guide design optimization and improve their reliability. We develop a computational model of the Less In More Out device, a fluidically actuated soft total artificial heart constructed from heat-sealed layers of woven fabric. Our model reproduces the nonlinear deformation, strain fields, and pressure-volume relationships measured in quasi-static experiments. Devices with fewer pouches deliver higher stroke volumes but exhibit up to 50% higher peak von Mises stresses. Fatigue analysis using a strain-life approach identifies heat-sealed seams and buckling regions as durability-limiting features. Our framework enables detailed evaluation of stress concentrations, buckling, and fatigue life, providing mechanistic insights that are difficult to obtain experimentally. It also offers a foundation for the optimization of artificial hearts and other fluid actuated fabric-based soft robotic systems.
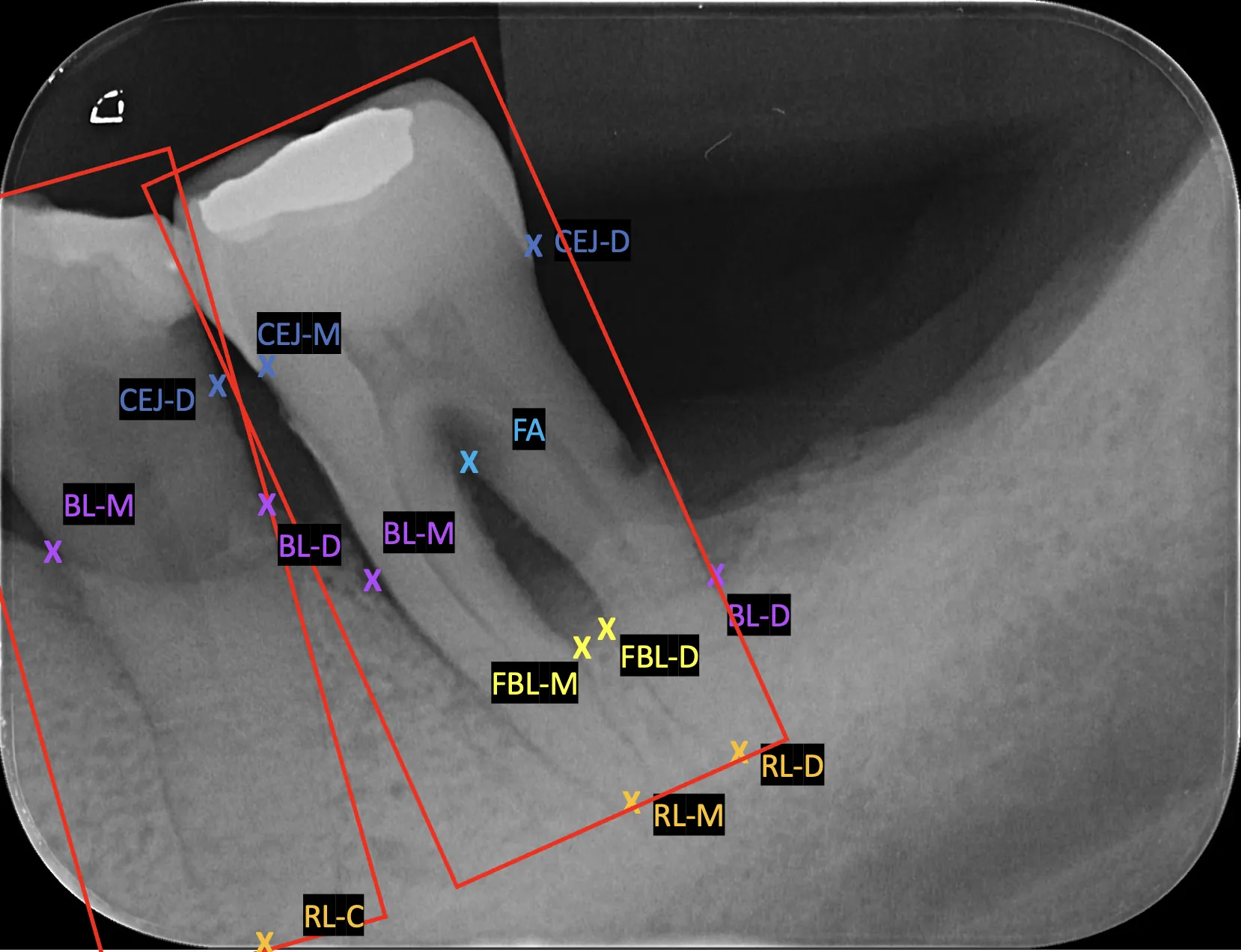
This study proposes a deep learning framework and annotation methodology for the automatic detection of periodontal bone loss landmarks, associated conditions, and staging. 192 periapical radiographs were collected and annotated with a stage agnostic methodology, labelling clinically relevant landmarks regardless of disease presence or extent. We propose a heuristic post-processing module that aligns predicted keypoints to tooth boundaries using an auxiliary instance segmentation model. An evaluation metric, Percentage of Relative Correct Keypoints (PRCK), is proposed to capture keypoint performance in dental imaging domains. Four donor pose estimation models were adapted with fine-tuning for our keypoint problem. Post-processing improved fine-grained localisation, raising average PRCK^{0.05} by +0.028, but reduced coarse performance for PRCK^{0.25} by -0.0523 and PRCK^{0.5} by -0.0345. Orientation estimation shows excellent performance for auxiliary segmentation when filtered with either stage 1 object detection model. Periodontal staging was detected sufficiently, with the best mesial and distal Dice scores of 0.508 and 0.489, while furcation involvement and widened periodontal ligament space tasks remained challenging due to scarce positive samples. Scalability is implied with similar validation and external set performance. The annotation methodology enables stage agnostic training with balanced representation across disease severities for some detection tasks. The PRCK metric provides a domain-specific alternative to generic pose metrics, while the heuristic post-processing module consistently corrected implausible predictions with occasional catastrophic failures. The proposed framework demonstrates the feasibility of clinically interpretable periodontal bone loss assessment, with potential to reduce diagnostic variability and clinician workload.
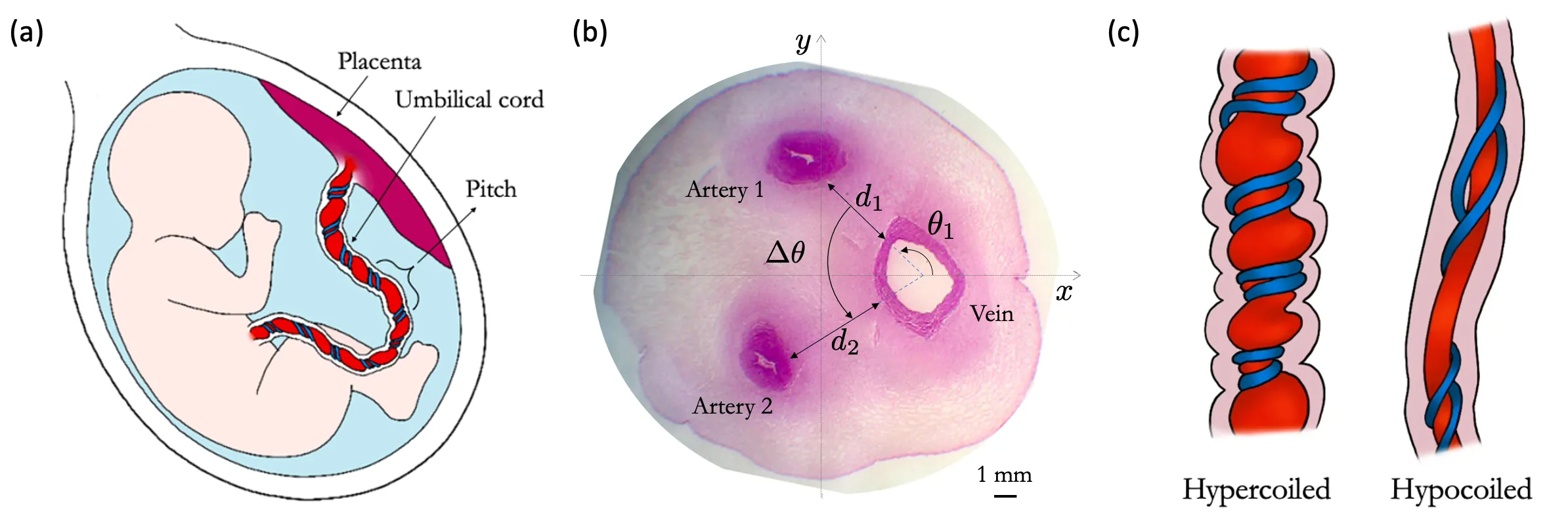
The umbilical cord plays a critical role in delivering nutrients and oxygen from the placenta to the fetus through the umbilical vein, while the two umbilical arteries carry deoxygenated blood with waste products back to the placenta. Although solute exchange in the placenta has been extensively studied, exchange within the cord tissue has not been investigated. Here, we explore the hypothesis that the coiled structure of the umbilical cord could strengthen diffusive coupling between the arteries and the vein, resulting in a functional shunt. We calculate the diffusion of solutes, such as oxygen, and heat in the umbilical cord to quantify how this shunt is affected by vascular configuration within the cord. We demonstrate that the shunt is enhanced by coiling and vessel proximity. Furthermore, our model predicts that typical vascular configurations of the human cord tend to minimise shunting, which could otherwise disrupt thermal regulation of the fetus. We also show that the exchange, amplified by coiling, can provide additional oxygen supply to the cord tissue surrounding the umbilical vessels.
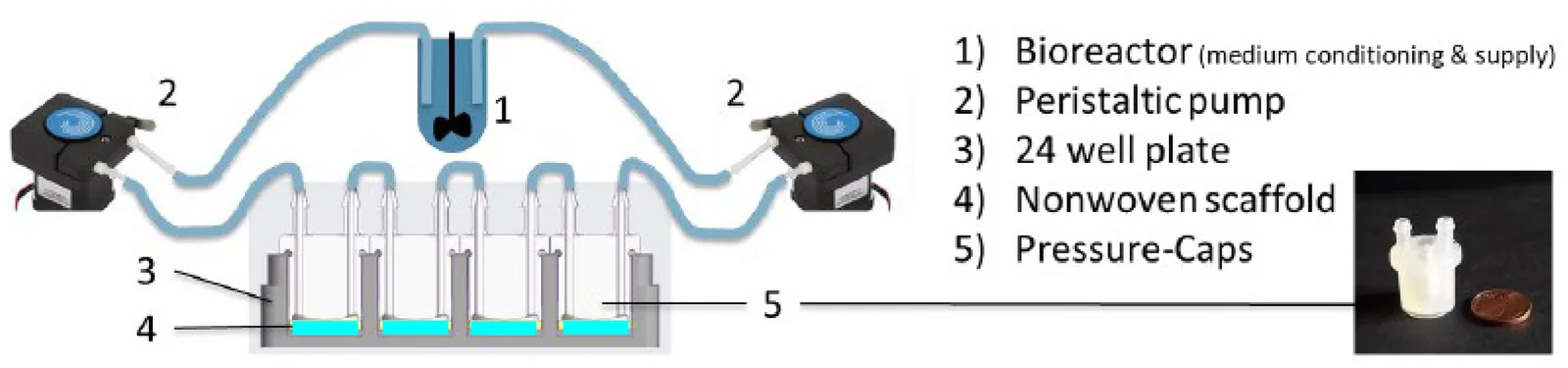
We develop a model the dynamics of human mesenchymal stem cells (hMSCs) and chondrocytes evolving in a nonwoven polyethylene terephtalate (PET) scaffold impregnated with hyaluron and supplied with a differentiation medium. The scaffold and the cells are assumed to be contained in a bioreactor with fluid perfusion. The differentiation of hMSCs into chondrocytes favors the production of extracellular matrix (ECM) and is influenced by fluid stress. The model takes deformations of ECM and PET scaffold into account. The scaffold structure is explicitly included by statistical assessment of the fibre distribution from CT images. The effective macroscopic equations are obtained by appropriate upscaling from dynamics on lower (microscopic and mesoscopic) scales and feature in the motility terms an explicit cell diffusion tensor encoding the assessed anisotropic scaffold structure. Numerical simulations show its influence on the overall cell and tissue dynamics.